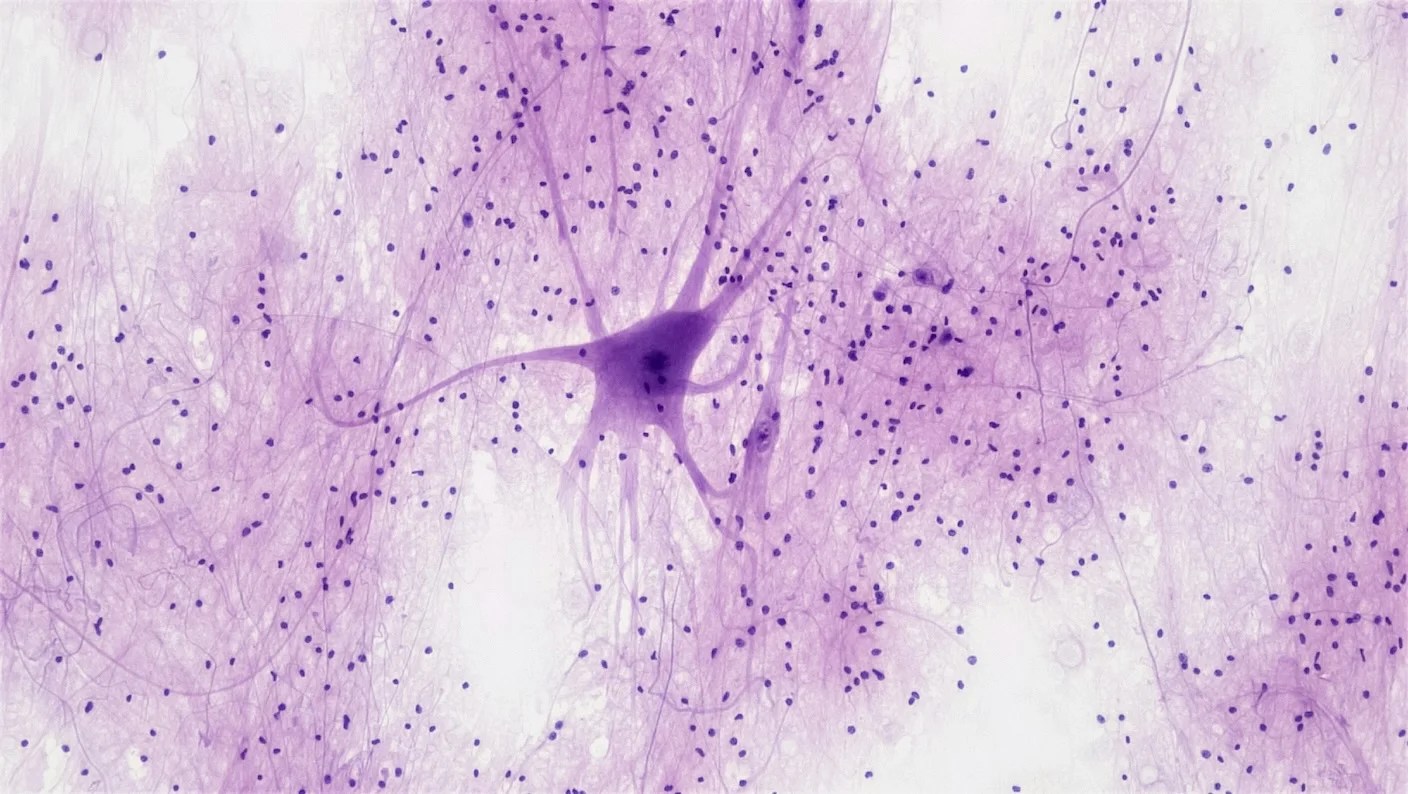
Brain cells are like tiny trees. They have an intricate web of roots that take in signals and a trunk that passes these signals to branches dotted with hubs called synapses, where the messages are shuttled to neighboring neurons.
It’s a very loose analogy. But it may be more accurate than neuroscientists previously thought.
At eye level, trees seem to grow alone, physically separated from other nearby trees. But under the soil, their roots are covered in a fungus with tiny thread-like channels. These weave tree roots into a vast web called the “mycorrhizal network.” Through these physical connections, trees can share water, nutrients, and chemical signals such as hormones—allowing them to communicate in what’s been dubbed a “woodwide web.”
Neurons may have a similar network. A new study imaging mouse and human brains discovered dynamic nanotube tunnels connecting dendrites, the roots of neurons. These wispy structures seemingly spawn from any point on the vast dendritic network and dissolve in minutes or hours.
Scientists don’t yet know exactly what they do. But the nanotubes can transfer electrical signals between neurons, a feat usually only attributed to synapses. They also allow neurons to share proteins, including those related to Alzheimer’s disease.
“The discovery suggests that the current understanding of the brain’s organization may be incomplete, overlooking a hidden layer of connectivity,” wrote Dimitri Budinger and Michael Heneka at the University of Luxembourg, who were not involved in the study.
Living Tunnels
Nanotubes are common in nature. Bacteria notoriously extend their membranes into tubes to share genetic material with their neighbors. This networking makes it easy to rapidly spread genes that are beneficial to the bugs, including those conferring antibiotic resistance.
Mammalian cells do it too. Around two decades ago, one team noticed fragile, membrane-like tubes spontaneously connecting rat kidney cells in a dish. Although both sides of the nanotube highways are open like a straw, they have surprisingly precise rules on cargo regulation. For example, some allow cells to transport select organelles—relatively self-contained components with specific functions inside cells—but only in one direction. Other cargo, such as proteins floating inside the cell’s watery interior are completely banned.
Scientists later discovered these elusive nanotunnels in a variety of other cell types grown in petri dishes, such as immune cells, cancer cells, and stem cells. The tunnels helped regulate viral infection, the spread of cancer, and organ development. Earlier this year, a group observed nanotubes in living zebrafish embryos. What job the nanotubes do seems to depend on the cell and tissue type, but they share similarities in their structural makeup and transient nature.
Telltale signs also suggest they help the brain stay healthy, at least in a dish. As the toxic proteins involved in Parkinson’s and Alzheimer’s disease accumulate, neurons form nanotubes that reach out to microglia, the brain’s immune cells. Called tunneling nanotubes, or TNTs, the newly built thoroughfares shuttle toxic proteins from neuron to microglia. In exchange, the microglia donate healthy mitochondria to the damaged neurons for an antioxidant boost.
“It was thrilling to observe that microglia play an active role in maintaining neuronal health and supporting neurons in times of need,” study author Hannah Scheiblich, who worked with Heneka on the project, said in a press release at the time.
Scientists are studying TNTs in other brain diseases, such as stroke and brain cancer. But observing them in the brain has been a headache. The tunnels are incredibly thin—a fraction of a human hair—and extremely fragile. Under conventional microscopy they easily get lost in the dense, chaotic tangle of neuronal branches.
Forest for the Trees
In the new study, the team combed through a large collection of electron microscope images of the mouse and human brain. Here they found signs of curious nanotunnels similar to TNTs. But they weren’t identical. For one, the new tubes connected dendrites—the neuron’s roots that take in signals—rather than sprouting from the longer trunk of a neuron. They were also less than one-third the length of TNTs.
The team next imaged wafer-thin living mouse brain slices and cultured human neurons with super-resolution microscopy. Using machine learning, they teased the slender structures apart from the relatively heftier neural branches and observed them.
Similar to TNTs, dendritic nanotubes are made mostly of a structural protein called actin. They’re highly dynamic, springing up and dissolving in a matter of minutes to hours.
The short timespan could impact how neurons work. Our brain cells transmit messages in three main ways. Synapses are the mainstay. These highly sophisticated “hubs” convert electrical signals into chemical messengers to pass on information. Gap junctions offer a rarer but speedier route: They rely solely on electrical signals. The third are like “spaceships” that neurons use to launch a variety of biomaterials to other nearby cells that influence their function.
Dendritic nanotubes seem to be a jack of all trades. They transmit electrical signals in the form of calcium, which neurons need to activate. When they artificially raise the amount of calcium in one neuron, neighboring neurons also register a boost. Adding a chemical that destroys the nanotubes partially blocks the effect.
Dendrites are mini-computers on their own, with synapses processing incoming signals in parallel and then shuttling the results to other parts of the cell for more processing. Nanotubes seem to operate independently of synapses. They create a network that could alter the activity of dendrites and allow neurons to share information outside the usual synaptic routes.
“What makes dendritic nanotubes conceptually exciting is that they expand the repertoire of known forms of communication among neurons,” wrote Budinger and Heneka.
Friend or Foe
Unlike TNTs, dendritic nanotubes are closed at both ends. While scientists are not exactly sure how and why this happens, the quirk could help regulate the transport of proteins—including harmful ones. When the team added human amyloid-beta—a protein involved in Alzheimer’s—to a single mouse neuron in a petri dish, it rapidly spread to other neurons. The transfer was nipped in the bud by destroying the nanotubes.
The wispy tunnels also showed up in a mouse model of Alzheimer’s. Their connectivity increased in the front part of the brain at three months of age—the rough equivalent of young adult in human years—and well before there were any signs of toxic amyloid-beta clumps.
But the effects were nuanced. Computer simulations supported the idea that the nanotubes contribute to amyloid-beta spreading but only at low doses, which could help dilute the toxic burden for a single cell. At high doses, however, the tunnels disintegrate and sequester the clumps inside infected cells, potentially to keep them from spreading further.
These results only scratch the surface. The team is exploring which cargo—specific proteins, RNA molecules, or organelles—are preferentially transported, how their abundance alters during aging and disease, and if they intersect with classic TNTs.
Still, the discovery “underscores that the brain’s connectome, the complete map of all the neural connections in the brain, is more than a wiring diagram of synapses” and should include these transient, nanoscale links that come and go, wrote Budinger and Heneka. They’re the “fourth pillar of intercellular communication.”
Source link
#Mysterious #Web #Tunnels #Connects #Brain #CellsLike #Network #Trees #Forest